Biopharmaceuticals
With the continuous growth of global pharmaceutical demand, the pharmaceutical industry faces increasingly stringent technical standards and regulatory pressures. Especially in the production and quality control processes, the requirements of international good manufacturing practices (GMPs) are gradually increasing, imposing higher standards on production line efficiency, product quality stability, consistency, and traceability throughout the entire process. In this context, pharmaceutical companies pay particular attention to the precision, cleanliness, and adaptability of equipment to different drug forms when selecting equipment.
For the production of lyophilized powders, due to the high requirements for the protection of active ingredients and the stability of the formulation, filling equipment must strictly meet high cleanliness and precise dosing control requirements. This is not only a key technical aspect to ensure the safety and quality of drugs but also an important guarantee for the compliant operation of pharmaceutical companies.
Customer Challenge
Our customer is a large biopharmaceutical company focusing on the development and production of various drugs, including lyophilized powders. As market demand grows, the original filling equipment gradually becomes unable to meet the increasing production requirements, and some parts suppliers have discontinued production, making maintenance difficult. To ensure the continuity of production and the stability of drug quality, the customer realized the need to upgrade filling equipment promptly.
Lead Fluid Solution: Clean and Precise
Based on over ten years of deep accumulation in the fluid transfer field, Lead Fluid has a profound understanding of the stringent requirements pharmaceutical companies have in equipment selection. For the customer’s lyophilized powder production needs, Lead Fluid recommended the DS600-X series filling system combined with the DMD25-T pump head. This solution aims to meet the high precision, low pulsation, and high cleanliness filling requirements and has been widely used in many pharmaceutical companies domestically and internationally.
Lead Fluid DS600-X Series Filling System
01 High Precision Filling
The DS600-X series uses high-quality servo motors or stepper motors for driving, and the optimized operating system and pipeline design ensure that the error between each filling head is controlled within ±1%, ensuring the accuracy of each dose and the consistency of product quality.
02 High Efficiency and Stability
The DS600-X series is equipped with an intelligent correction system and an online micro-adjustment speed function, allowing users to adjust for flow changes caused by hose aging. Its stainless steel casing is corrosion-resistant and easy to clean, enhancing the durability of the entire machine. Each channel is equipped with a stop-filling function when the bottle is empty, ensuring the stability of long-term continuous production.
03 High Flexibility
The modular design allows the equipment to be flexibly adjusted according to production needs, adapting to filling requirements of different specifications and capacities. It can be paired with automatic filling production lines or operated manually.
Lead Fluid DMD25-T Pump Head
01 Low Shear, Clean and Pollution-Free
The DMD25-T low shear force design effectively protects the integrity of fluid components. At the same time, the closed pipeline ensures that there is no contact with pollution sources throughout the transportation and filling process, making it particularly suitable for lyophilized drug products requiring high cleanliness.
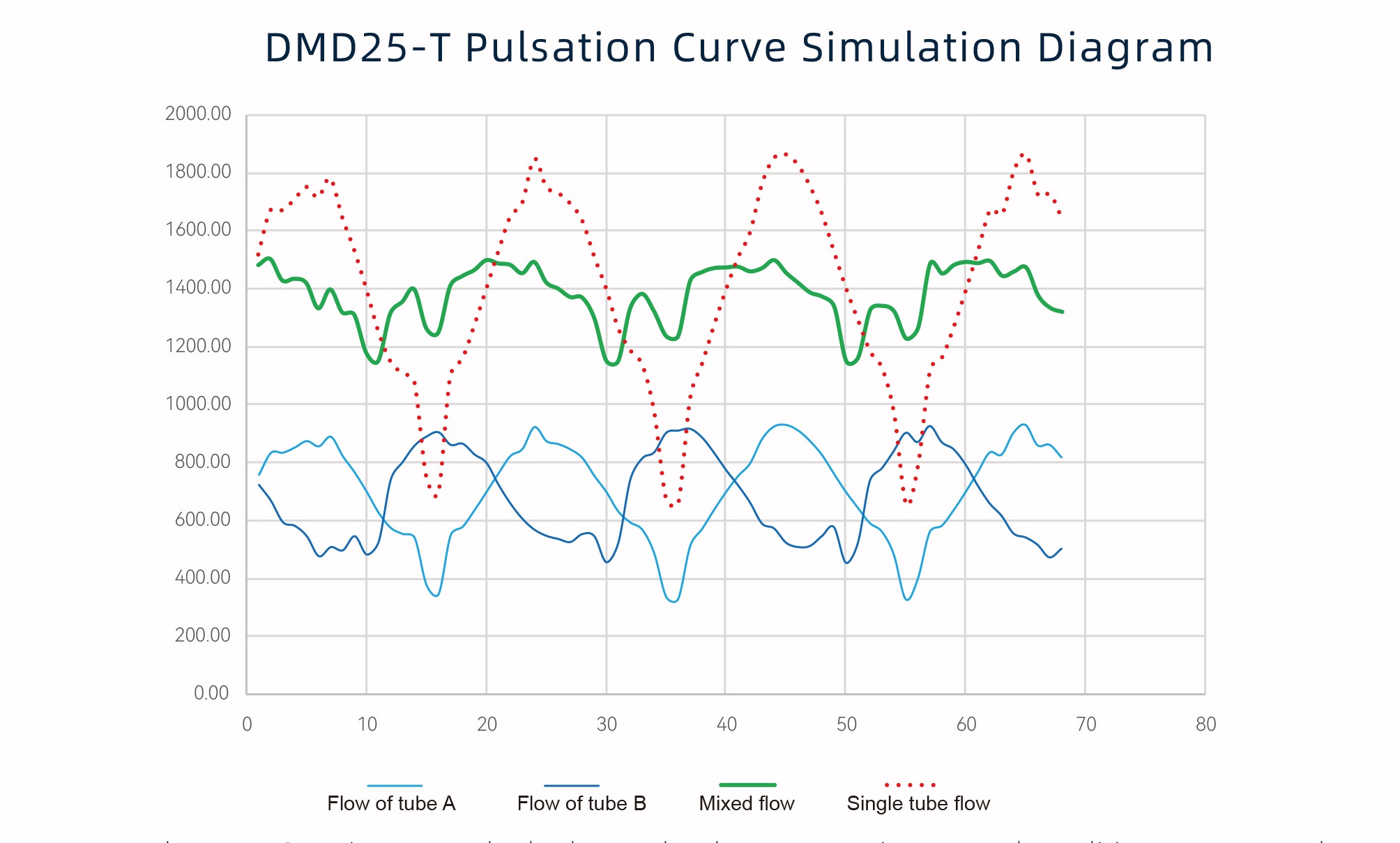
02 Low Pulsation Design, Precise Control
The DMD25-T uses phase compensation angles and a pressure release structure to significantly reduce pulsation during fluid transfer, making filling more stable and accurate, greatly improving product pass rates, reducing valuable raw material waste, and lowering production costs.
*The DMD25-T is an A+B dual tube-line; under the same environmental conditions, the pulsation curve of the mixed pipeline flow rate is compared with the single tube-line flow rate of the same flow rate.
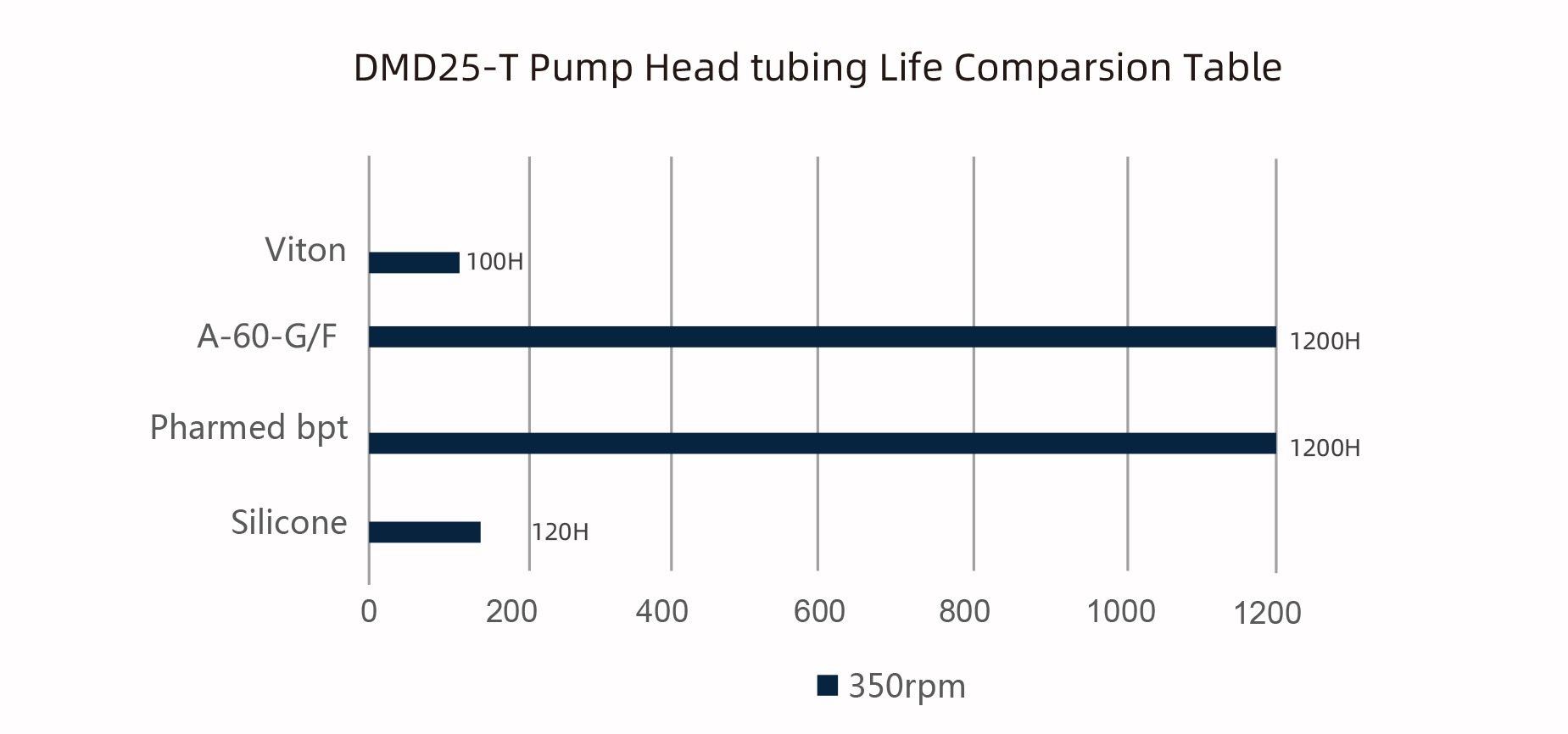
03 Extending Tube Life and Reducing Maintenance Costs
The DMD25-T uses imported high-quality spring materials, professionally calibrated to extend hose life and reduce downtime caused by frequent replacements. Compared with ordinary pump heads, it can maintain stable performance during long-term operation, lowering maintenance costs and increasing production efficiency.
*The above data are obtained from Lead Fluid laboratory tests using purified water under normal temperature and pressure. Actual service life may be affected by specific factors such as pressure, temperature, medium characteristics, tube batch, and wall thickness. Please contact Lead Fluid engineers for specific requirements to obtain better technical support.
04 Patent Design, Automatic Hose Centering, Stable and Efficient
The automatic tube centering structure of the DMD25-T has obtained international invention patents (patent number: US011319945/EP3674549). This design ensures that the pump tube remains in the optimal position every time it is installed, avoiding errors caused by pump tube deviation, making equipment operation more stable and further improving production line efficiency.
With its superior design, performance, and reliability, Lead Fluid has become a trusted partner for many pharmaceutical companies. We offer not only efficient and reliable products but also leverage our extensive industry experience and technical expertise to help companies navigate equipment upgrades. Lead Fluid will continue to advance clean and precise filling technology with the pharmaceutical industry, contributing to innovation in drug production worldwide.