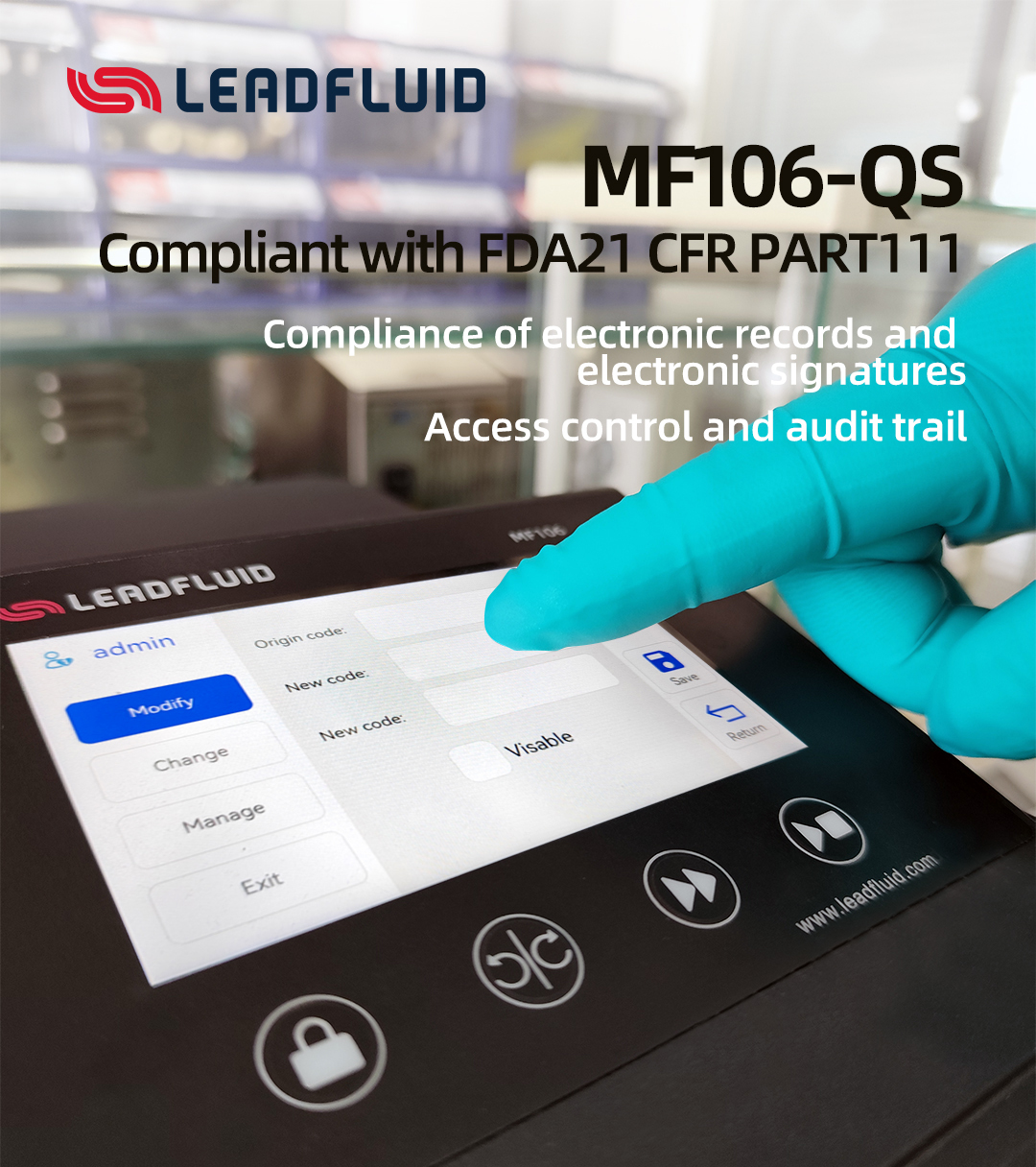
MF106-QS, the latest masterpiece in the Lead Fluid High Protection Series peristaltic pump! Specifically designed to meet the stringent standards of the biopharmaceutical industry! It not only continues the outstanding performance of its predecessor (MF106), but also features numerous upgrades in various aspects.
Compliance Assurance
● Electronic Signature Compliance: Built-in electronic signature functionality, compliant with FDA 21 CFR Part 11, ensuring data security and regulatory compliance.
● Permission Management: Flexible hierarchical permission settings, supporting ID and password policies, with secure and reliable operations.
● Data Traceability: Real-time recording throughout the process, supports export, complete and traceable data, facilitating audits. 
Pharmaceutical Preferred
● Follow the 3Q validation system and comply with the high standards of pharmaceutical processes and GMP, ensuring an efficient and compliant production process.
Optimize User Experience
●Convenient USB upgrade: Supports USB drive updates, reducing maintenance costs.
●User-friendly experience: Built-in help center with video guidance for quick mastery.
Performance Enhancement
Triple the computation speed, with faster response times.
Lead Fluid – Your reliable partner in biopharmaceuticals.
MF106-QS is coming soon! Contact Lead Fluid for more information!