Peristaltic pumps have been a traditional liquid-filling technology for biopharmaceutical manufacturers, but stricter validation requirements, new challenges, and design innovations have led pharmaceutical companies to consider peristaltic filling technology. About 80% of medical and pharmaceutical companies use peristaltic pumps, and they are used in all aspects. Lead Fluid peristaltic pump is accurate, durable, stable in delivery flow, continuously adjustable, has a small amount of delivery, and can also be used for small filling. The fluid is isolated in the pump tube and does not contact the outside to prevent pollution. The pump tube can be quickly replaced. The fluid can be reversed. The characteristics of dry operation and low maintenance cost constitute the main competitive advantage of the peristaltic pump, making it widely used in medical and pharmaceutical fields.
Pharmaceutical manufacturers show increasing interest in developing biopharmaceutical products, and observers believe these products could represent the industry’s future.
Visit BT601F peristaltic pump used in Pharmaceuticals industry
Biopharmaceutical development history
Biopharmaceuticals mainly refer to the use of research results and technologies in the fields of biology, medicine, etc., to extract raw materials from natural biological materials such as microorganisms, humans, animals, jobs, marine organisms, etc. Of medical supplies for prevention, treatment and diagnosis.
The development history of biopharmaceuticals is long, let us go through history together and relive every shining segment of technological progress.
1. Traditional biotechnology
1.1 Biological technology has been shining in wisdom since BC. In 6000 BC, the Babylonians used natural fermentation to brew beer, and my country’s Zhou Dynasty also used natural fermentation to make vinegar.
1.2 The earliest record of using biological materials as therapeutic drugs can be traced back to the Shennong’s Materia Medica of the Eastern Han Dynasty, which records the method of treating goiter with seaweed.
1.3 In 1931, Ernst Ruska entered a new stage in the biological research of objects as small as one millionth of a millimeter by developing an electron microscope.
 |
 |
 |
2. Modern biopharmaceutical development
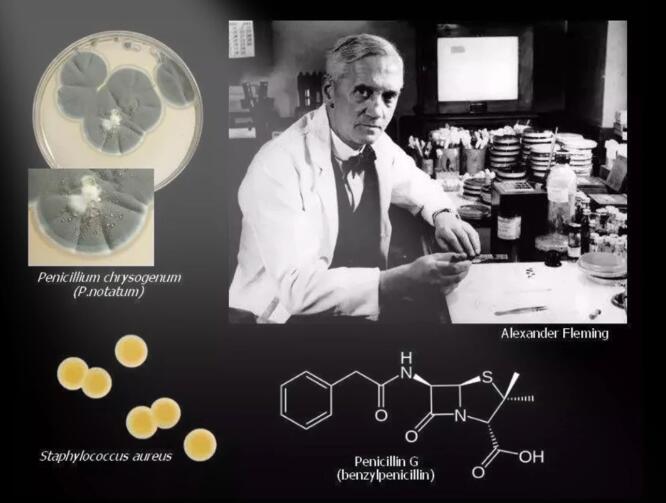
The 20th century entered the glorious era of biopharmaceuticals. One day in 1928, British bacteriologist Fleming piled all the bacterial culture medium on the bench in the corner of the laboratory before going on vacation. After returning from vacation. He found that one of the culture medium was accidentally contaminated by mold, and the staphylococcus around the mold was killed. He tried to purify this sterilizing material, but there were too many impurities and no success.
During World War II
Two British scientists, Florey and Chann, successfully isolated this bactericidal substance. This bactericidal substance is called “penicillin”. The advent of penicillin has saved thousands of wounded lives.
Since penicillin, the research and production of antibiotics have developed rapidly. So far, thousands of antibiotics have been discovered and invented by humans.
In the 1980s, the development of biopharmaceuticals opened the accelerator
On October 28, 1982, the U.S. FDA issued a letter of approval: the first genetically recombinant human insulin was officially launched. This revolutionary drug manufactured by genetic engineering has advanced the clinical application of insulin to an unprecedented high speed. With the advent of a new historical stage in the pharmaceutical industry.
1986 was a shining year for biopharmaceuticals. A variety of important drugs were launched one after another. Muromonab-CD3, the first mouse monoclonal antibody drug, was officially launched, which can be used to prevent acute organ rejection during kidney transplantation. In the same year, the first anti-tumor biotechnology drug interferon was launched on the market. In the same year, Merck Pharmaceuticals developed the world’s first recombinant genetically engineered hepatitis B vaccine, bringing the hepatitis B vaccine to thousands of households.
3. History of Biopharmaceutical Development in China
3.1 The initial phase
Before the founding of the People’s Republic of China, China Biopharmaceuticals was basically in its infancy. Only the Shanghai Yang’s Pharmaceutical Factory founded by Dr. Yang Shuxun was struggling to produce a small amount of biochemical drugs, such as oral hydrolyzed protein, vitamin C, liver injection and posterior pituitary injection, etc.
After the founding of the People’s Republic of China, Yang’s Pharmaceutical Factory was renamed Shanghai Biochemical Pharmaceutical Factory in 1985, becoming China’s first professional pharmaceutical factory.
With the “First Five-Year Plan” (1952-1957), the spring breeze of biopharmaceuticals began to blow into China.
In 1953, the North China Pharmaceutical Factory, once the largest antibiotic manufacturer in Asia, was completed and put into operation, completely ending China’s history of relying on imports of penicillin.
3.2 Development highlights
The first synthetic bovine insulin
After receiving the Nobel Prize in Chemistry for the analysis of the chemical structure of insulin, Nature published a review article saying: Synthetic insulin will be a distant thing, but far away China has officially started this “remote” thing-synthetic insulin.
The Shanghai Institute of Biochemistry, Chinese Academy of Sciences, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, and the Department of Chemistry, Peking University are united by three units, headed by Jingyi Niu, and composed of Yueting Gong, Chenglu Zou, Yucang Du , Aixue Ji , Qiyi Xing, You Wang, Jiecheng Xu and others Collaboration group.
On the basis of predecessors’ research on insulin structure and peptide chain synthesis methods, they began to explore the use of chemical methods to synthesize insulin.
After careful research, they completed the total synthesis of crystalline bovine insulin on September 17, 1965. Through strict identification, its structure, biological vitality, physical and chemical properties, and crystal shape are exactly the same as those of natural bovine insulin.
This is the first time in the world that a protein with the same chemical structure and complete biological activity as a natural insulin molecule has been artificially synthesized.
4. China’s first genetic engineering innovative drug
British virologist Eric Isaacs discovered during his research on influenza virus that he reused chicken embryo chorioallantoic membrane to study the phenomenon of influenza interference. He learned that virus-infected cells can produce a factor that acts on other cells to interfere with the virus. Copy it so named interferon.
Interferon has broad-spectrum anti-virus, anti-tumor and immune functions.
In order to use interferon in clinical drugs, Chinese academician Yunde Hou led the team to repeat experiments and finally cloned the interferon α1b gene with China’s independent intellectual property rights for the first time in 1982, and successfully developed first genetic engineering innovative drug-recombinant human Interferon α1 is a national first-class new drug product created in the world, creating a precedent for the research and development of innovative genetic engineering drugs in China..
Enter the 21st century
With the introduction of a series of new drug policies, China’s biological research and development has entered a stage of vigorous development. China is currently catching up in the third and fourth phases of coexistence, in hot areas such as CAR-T, PD-1 inhibitors and bispecific antibodies. Gradually narrowing the gap with developed countries.
Lead Fluid hopes to join hands with more practitioners in the biopharmaceutical field to jointly promote technological innovation in the biopharmaceutical field, and believes that through the continuous development of biopharmaceutical technology, more mysteries of human life will also be overcome.